*** If you have a registered OTC facility with the US FDA – this update will be of interest to you ***
Cosmetics Alliance was informed by some members earlier today of an e-mail they received regarding outstanding invoices in relation to the Over-the-Counter Monograph Drug User Fee Program (or OMUFA) in the US. The manner in which the e-mail was communicated, as well as the somewhat awkward payment and contact details, gave the impression that this was perhaps a phishing scam. We contacted our colleagues at the US Personal Care Products Council (PCPC) to verify whether they were aware of these activities and could confirm if this Notice is in fact legitimate. The PCPC has confirmed that they too have had similar queries from their membership regarding these e-mail notices.
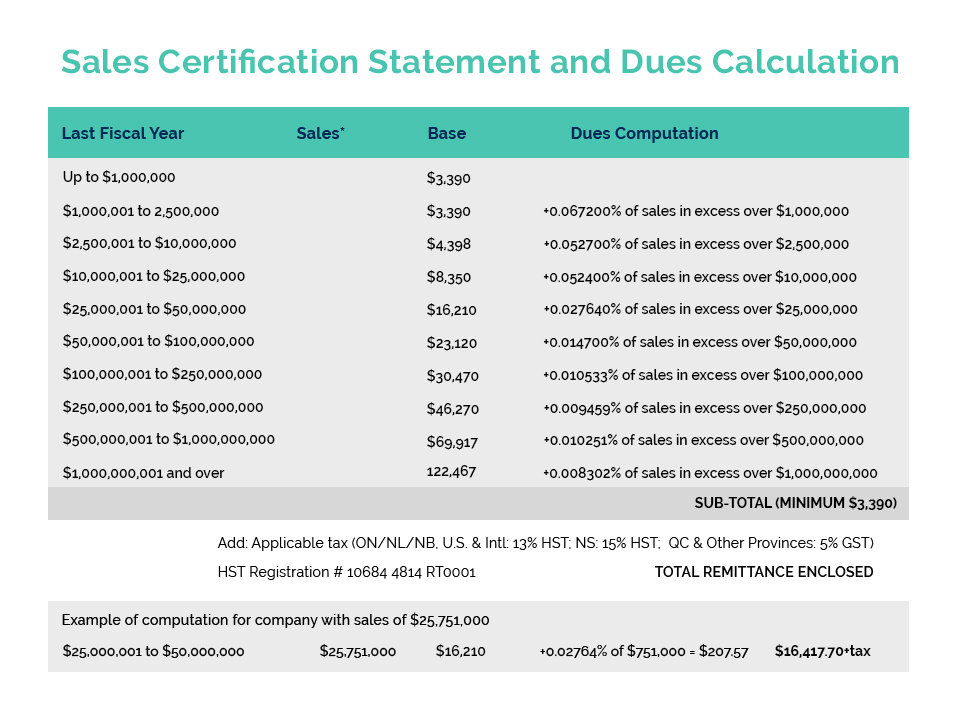
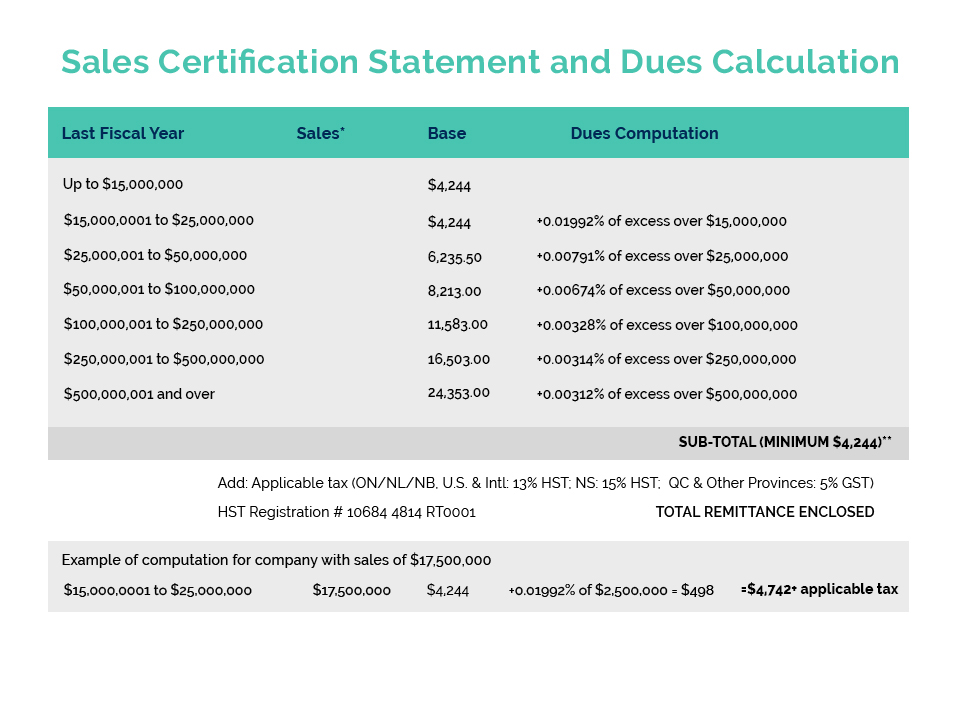
Based on discussion with PCPC, it appears that these activities are likely legitimate. As we understand it, the fees outlined in this Notice are not new and are a function of the OTC User Fee Reforms in place in the US (published Mar 27, 2020). The Notice makes reference to the Fiscal Year 2021 (FY2021) User Fee Rates for OTC drug facilities and OTC contract manufacturing facilities (under Section 744M of the Federal FD&C Act) as registered with the US FDA. Details regarding OMUFA can be found at: 2021-06361.pdf (federalregister.gov) with corresponding FY2021 User Fee Rates detailed at: Federal Register: Fee Rates Under the Over-the-Counter Monograph Drug User Fee Program for Fiscal Year 2021.
What is not clear is who received this Notice. Registered facilities should have received an original invoice from the US FDA around March 26, 2021 (which was the official publication of the FY2021 Fees Notice). Payment was due 45 days following this official publication (May 10, 2021). If you received this invoice, and have processed payment; you should NOT have received this Notice of Overdue payment. If you are in receipt of such a Notice, it is likely that you are a registered facility and that this is a follow-up Notice, indicating that your invoice is presently in arrears.
Based on discussions with PCPC, we understand that the intent of this Notice is to serve as a courtesy reminder that the payment deadline has been missed before escalating collections. The next step would be for the FDA to send out formal overdue notices, at which time companies that have still not paid their invoices will be in arrears (with facilities formally published on the User Fees Arrears List – which is a public notice process). So there is still time to act.
RECOMMENDATION – WHAT CA CANADA MEMBERS SHOULD DO IF YOU RECEIVE SUCH A NOTICE
Do you own a facility that is registered with the US FDA for manufacturing OTC drug products (or contract manufacturing of OTC drug products)?
IF YES, it is likely that you have an outstanding invoice with the US FDA:
- You can check on-line to determine if your invoice is outstanding at the FDA’s public facing database https://www.fda.gov/industry/fda-user-fee-programs/over-counter-monograph-user-fee-program-omufa under the User Fee Lists dropdown menu.
- Verify if you received an original invoice from the US FDA in March.
- If you did not receive an original invoice, we would recommend that you contact the User Fees Staff at the US FDA to inquire as to the status and legitimacy of your invoice (and notify the US FDA that you were never in receipt of the original invoice). Phone: (301) 796-7200 / Email: userfees@fda.gov
- Make arrangements for payment, as required
IF NO, you may be in receipt of this notice in error
- Contact the User Fee Staff at the US FDA to notify them that you are do not have a registered OTC facility in the US. Phone: (301) 796-7200 / Email: userfees@fda.gov
RESOURCES
You can access the US FDA User Fee Program Web page at: https://www.fda.gov/industry/fda-user-fee-programs
View a detailed Q&A on the US FDA User Fee Program here: https://www.fda.gov/industry/over-counter-monograph-user-fee-program-omufa/other-omufa-fee-related-questions
If you have any questions regarding this program, we would recommend reaching out to US PCPC (if you are members) OR to the US FDA.
Of course, we would be pleased to discuss these developments in further detail with you. Please do not hesitate to contact us at regulatory@cosmeticsalliance.ca