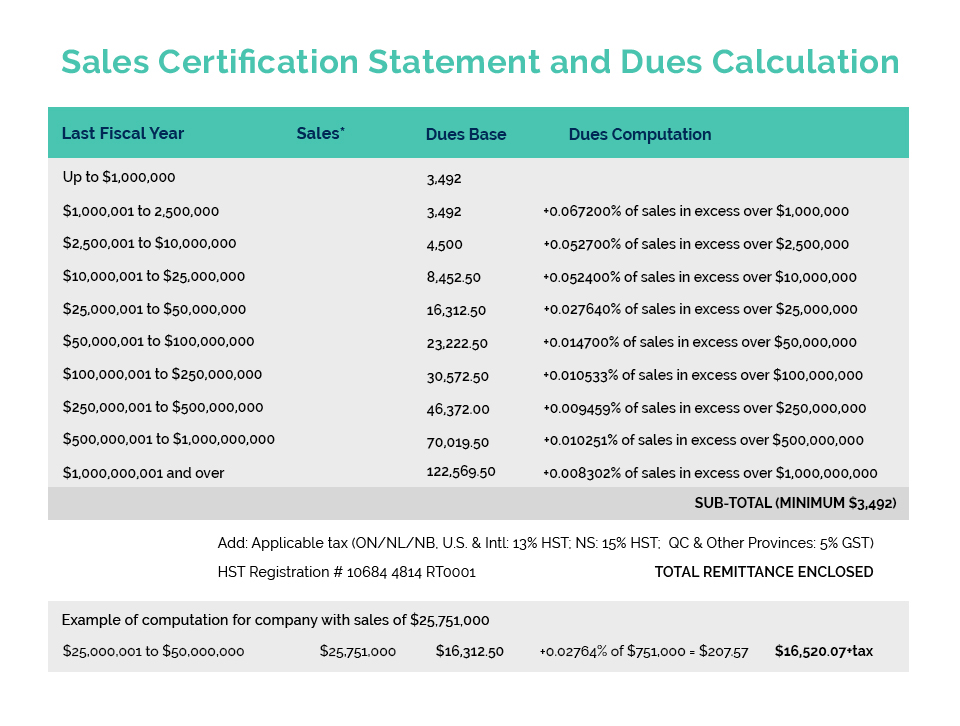
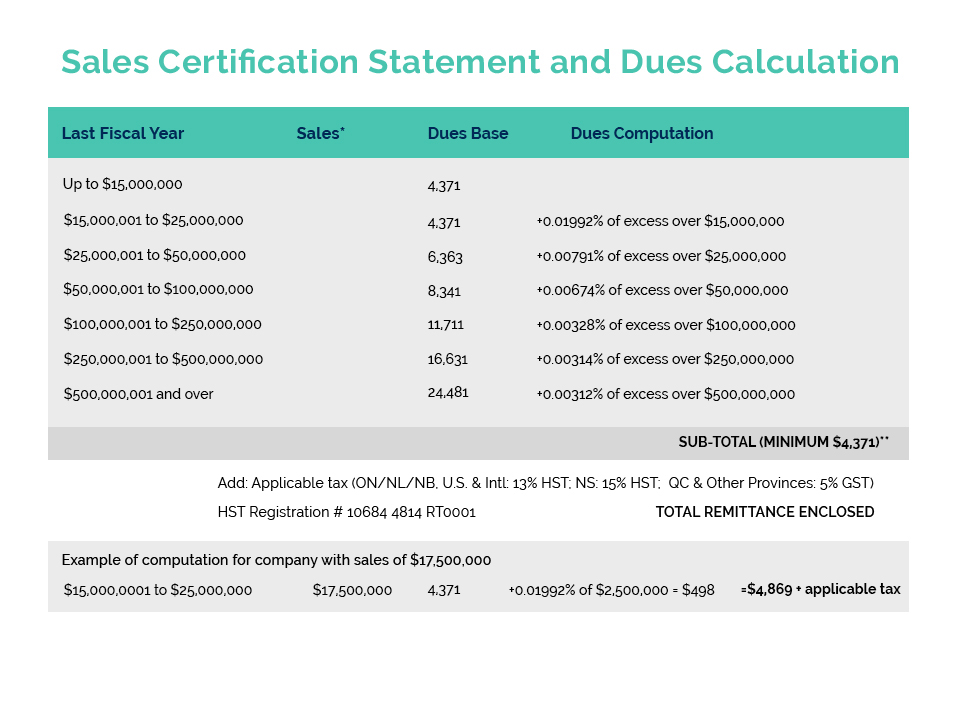
On February 24th, the Natural & Non-prescription Health Products Directorate (NNHPD) held its winter Multilateral Meeting virtually with its key industry associations. Filling CA’s three seats at the “virtual table” were Beta Montemayor, Richard Parcels and Gulnara Gabidullina (Vice Chair of CA’s Product Compliance & Market Access Committee).
NNHPD’s newly appointed Director General, Natalie Page, opened the meeting with her team and officials from the Inspectorate (ROEB) providing the updates on the following key items:
• COVID backlog and the measures in place to clear the back-log and resume normal performance standards now that the influx of pandemic-related registrations such as hand-sanitizers has subsided
• Forward Regulatory Plan and assurances of Health Canada’s post Covid-19 commitment to the Self-Care Framework as a priority
• NHP Inspections and work towards establishing an NHP GMP Inspection Program Pilot that moves away from from the prescriptive drug inspection model, in keeping with the principles of Self-Care
• Cannabis Science Advisory Committee which will provide independent scientific and clinical advice to support the Department’s consideration of appropriate safety, efficacy, and quality standards for health products containing cannabis
Some important staff changes at NNHPD were also discussed. Robin Churchill, who was the acting DG, will be moving to take on a senior position at the Food Directorate, while Amanda Moir will assume responsibilities as Associate Director General. Amanda has led the Self-Care Framework Project team over the past few years and her new leadership role will be helpful in advancing the Framework. CA wishes to express its appreciation for the excellent work of Robin Churchill at NNHPD during her time in the Directorate, and especially for her outstanding efforts during the pandemic. We are also looking forward to working with Amanda in her new role.