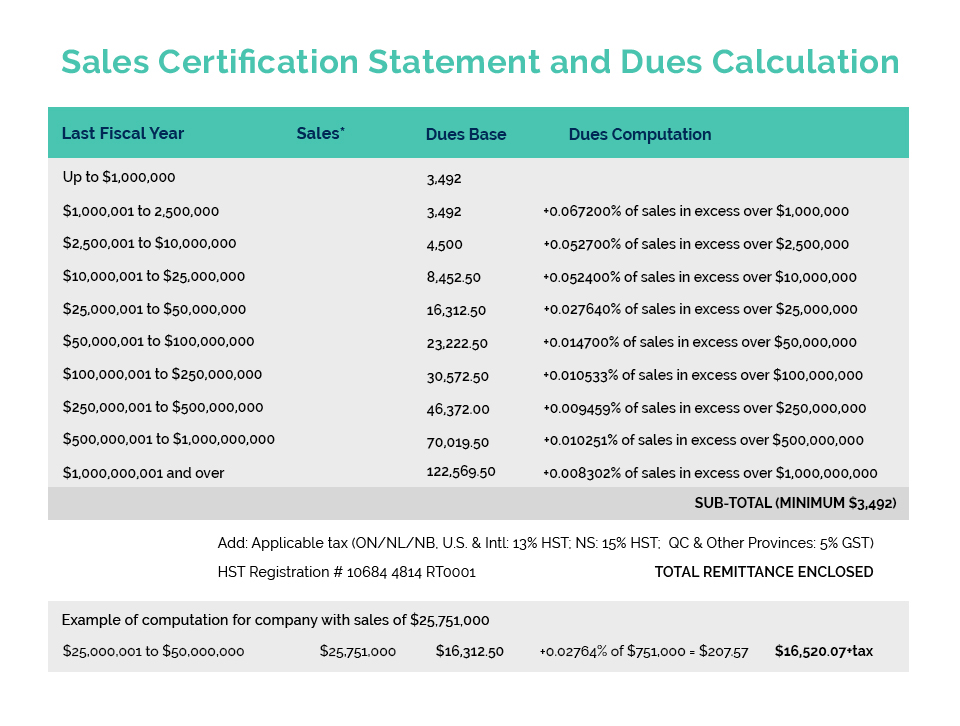
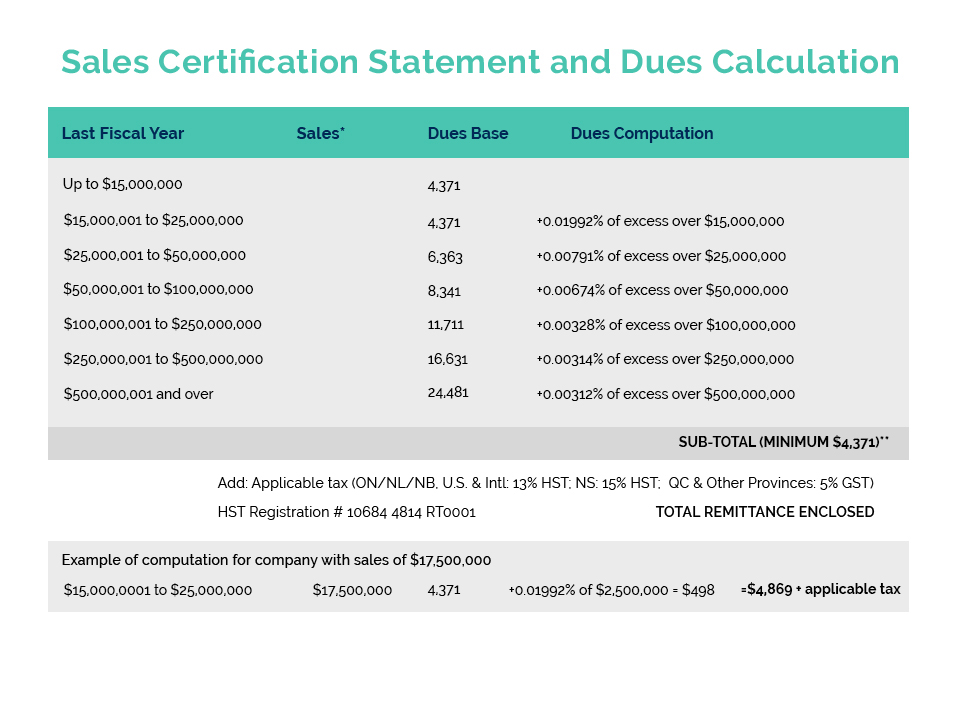
New Applications No Longer Accepted
As of January 15, 2021, Health Canada stopped accepting new applications for all hand sanitizers through their Covid-19 Interim Measures.
Currently Approved Products Continued – With 60 Day Notice Before Expiry
Hand sanitizers previously accepted under the interim measure continue to be allowed to be imported and/or sold by companies that previously received acceptance correspondence from Health Canada (HC). HC will provide 60-day advance notice to companies that received a confirmation email of acceptance through the interim measure, outlining the process to follow when the interim measure for hand sanitizers expires.
Drug-based Hand Sanitizers & Interim DEL Provisions
Companies conducting activities related to drug-based hand sanitizers using a Drug Establishment Licence (DEL) exemption provided in a previous Interim Order, are reminded to follow the requirements to apply for a DEL before September 1, 2021 as outlined in DEL Bulletin No. 109 sent on March 2, 2021. Further details on obtaining a DEL to conduct licensable activities related to these products can be found in the designated hand sanitizers section of the exceptional importation and sale of drugs in relation to COVID-19.
Alcohol-based Hand Sanitizers (Natural Health Products) – Temporary Site Licenses
For alcohol-based hand sanitizers (natural health products) temporary COVID-19 Site Licences (SL) remain valid. As such, as previously communicated to all COVID-19 Site licence holders in August 2020, at this time, no action is required of you (see attached SL holder bulletin from August 2020). Temporary COVID-19 SLs remain valid until you are advised otherwise, when the unprecedented demand created by the public health emergency no longer exists. Health Canada will provide all temporary COVID-19 SL holders with 60-days advanced notice of when the interim measures associated with the temporary COVID-19 SL will cease to be in effect.
General Information
HC is exploring timing and details for when hand sanitizer related interim measures will end and will communicate appropriately to all implicated stakeholders including all COVID-19 site licence holders with products accepted through the interim measure.
CA will continue to monitor developments and keep members updated.