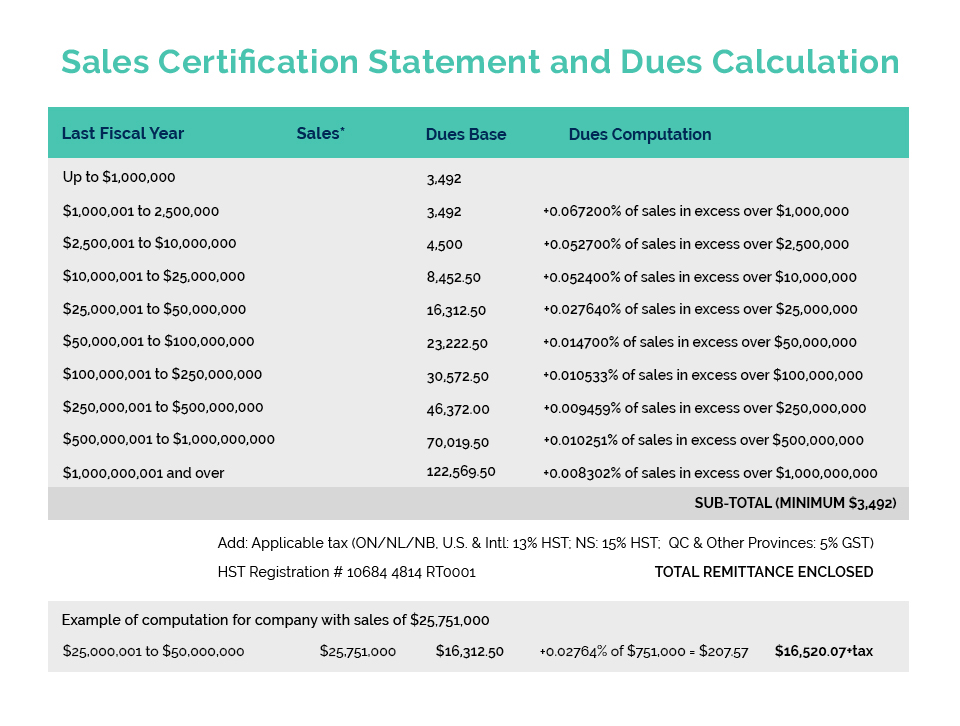
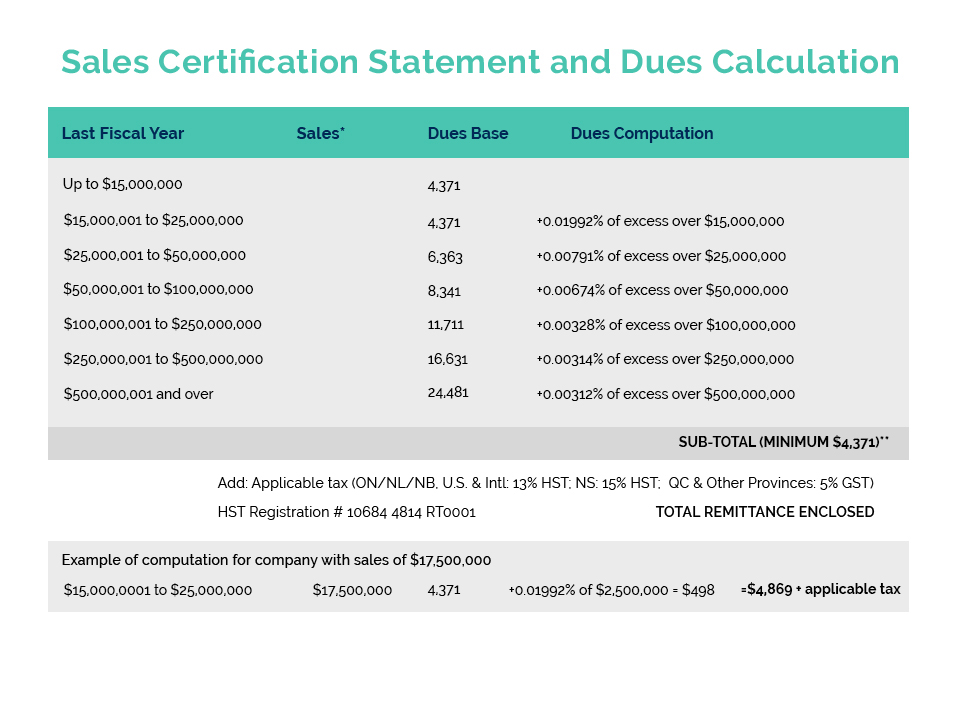
CA recently held another Interactive Training Session for our members entitled “Personal Care Product Ingredients and the Canadian Environmental Protection Act (CEPA)”. Our co-presenters Michele Richardson (Icon Life Sciences) and Dan Bastien (Intertek) provided an informative introductory course on Canada’s complex and often challenging environmental protection legislation.
Participants learned about our industry’s responsibilities under CEPA for cosmetics, drugs and natural health products, Canada’s Chemicals Management Plan, stakeholder notifications (NSNs) and mandatory survey requirements, and more. Training Certificates were issued to every individual who successfully completed the quiz. These CA certified documents can be readily added to an individual’s training file!
Stay tuned for upcoming sessions in our training and webinar series.
About the Trainers
 Michele Richardson, B.Sc., MBA
Michele Richardson, B.Sc., MBARegulatory Affairs, Environmental
Michele is co-Chair of the New Substances Notification subcommittee of the CEPA Industry Coordinating Group. She was a member on the US/Canada Regulatory Cooperation Council’s Technical Working Group and sat on the Biotech Industry Government Working Group for Organisms. Ms. Richardson has facilitated workshops with officials from Environment and Climate Change Canada, Health Canada and the US EPA.
 Dan Bastien
Dan BastienHealth, Environmental & Regulatory Services (HERS) Associate Director Chemicals Group
Intertek Health Sciences Inc.
Dan Bastien is the Associate Director of the chemical group of the Health, Environmental & Regulatory Services of Intertek Health Sciences Inc. Dan is a subject matter expert in Canada with specific experience in the Chemical Management Plan. Prior to joining Intertek, Dan managed the Substances Management Information Line and delivery of client services for the Chemical Management Plan of Environment and Climate Change Canada. His main responsibilities were to provide regulatory advice to industry stakeholders on the obligations to submit notifications of new chemicals, polymers and product of biotechnology substances in Canada. Dan was also responsible in the development and delivery of training materials, webinars and written material such as pamphlets, flyers and posters. He also designed and then managed the substances website.
Dan is well known for his ability to effectively communicate the impacts of the regulatory environment on the chemical industry. He has formed strong relationships with other Government stakeholders in the Americas, Europe and Asia-Pacific. He is frequently invited to present his advice and experience to a wide range of SME, large multi-national audiences and international authorities. His regulatory experience and his strong knowledge of the North American Chemical Industry, makes Dan uniquely qualified to provide practical service to help industry stakeholders understand and meet their regulatory requirements.