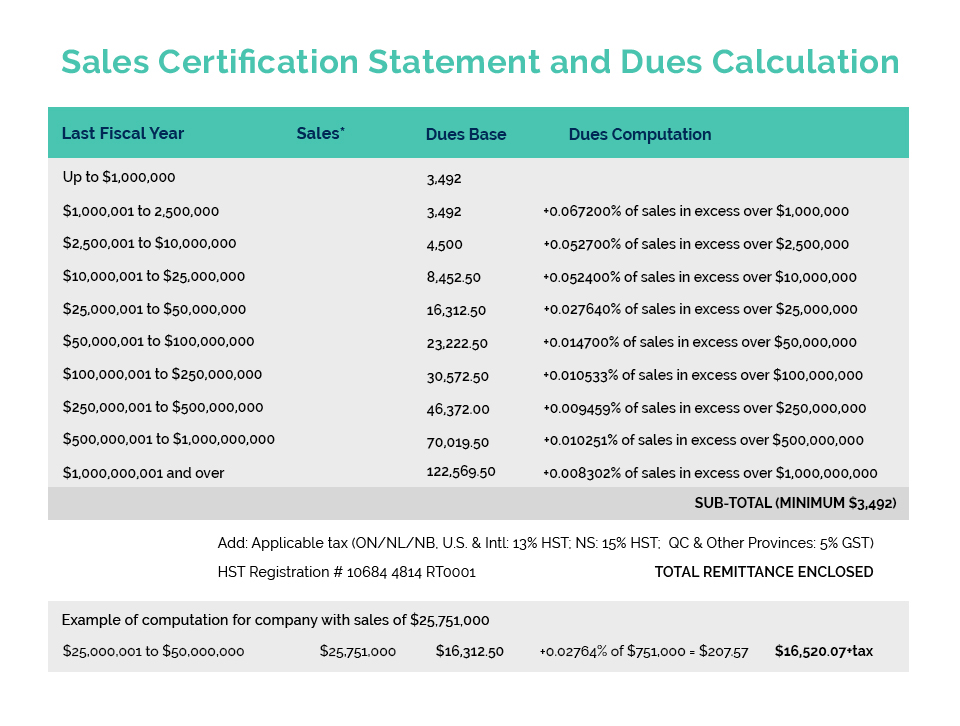
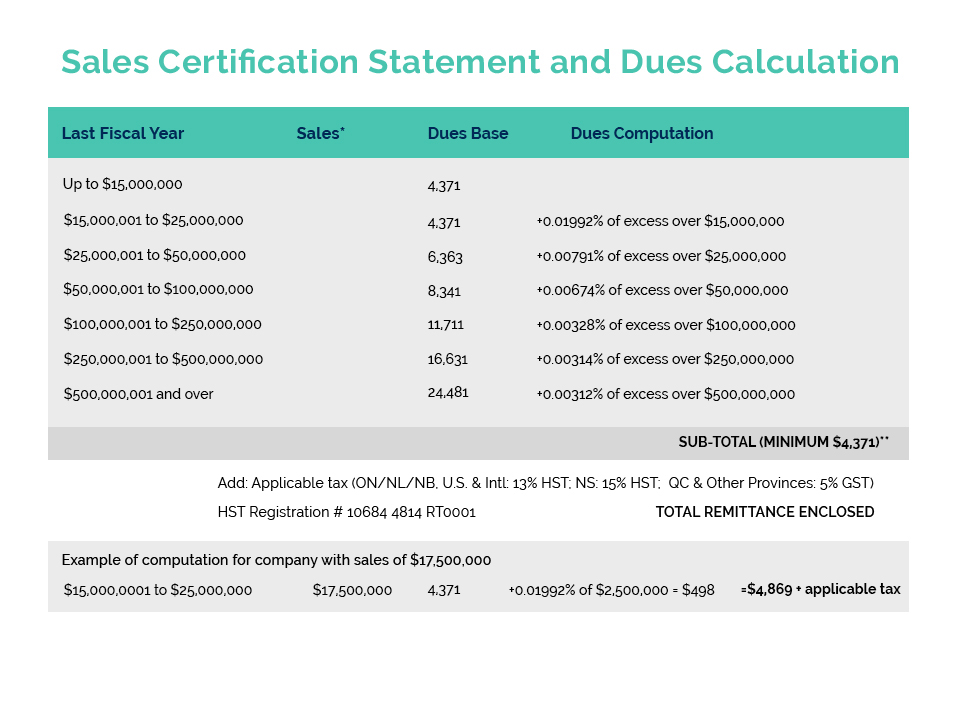
Cosmetics Alliance Canada, the U.S. Personal Care Products Association, and Mexico’s CANIPEC recently wrote a joint letter to our respective government agencies to convey our collective support for the Canada-United States-Mexico Agreement (CUSMA, the new NAFTA) and appreciation for the Cosmetics Annex which will align good regulatory practices and result in significant benefits for our manufacturers and consumers in all 3 countries.
The intent of the letter, which can be read HERE, was to bring attention to the outstanding action items identified in the Cosmetics Annex and the U.S.-Canada Appendix that require further work and to ensure that it is on the agenda of regulators. More specifically, this includes:
• Health Canada aligning its tamper-evident packaging for NHP’s with both it requirements for DIN’s and the US requirements for OTC’s
• The US FDA accepting the option for the sole use of some 57 INCI “Latin-based” trivial names without requiring that their English names be included (i.e. aqua by itself rather than having to include water). It has been estimated that this adds over $113M per year to the cost of US exports to Canada alone.
• Both Health Canada and the US FDA agreeing to common terms (and the use of italics), or mutually accepting each other’s rules, for Drug Facts labelling. (i.e. physician vs. doctor)
The letter was addressed to the three CUSAM Commissioners responsible for the implementation of the agreement and was intended to put a focus on both Health Canada and the US FDA to address these outstanding issues as well as to make clear industry’s expectations. The Canadian Commissioner is Trade Minister Mary NG whose Markham area riding includes many of our member companies and who has been a strong advocate for our industry.