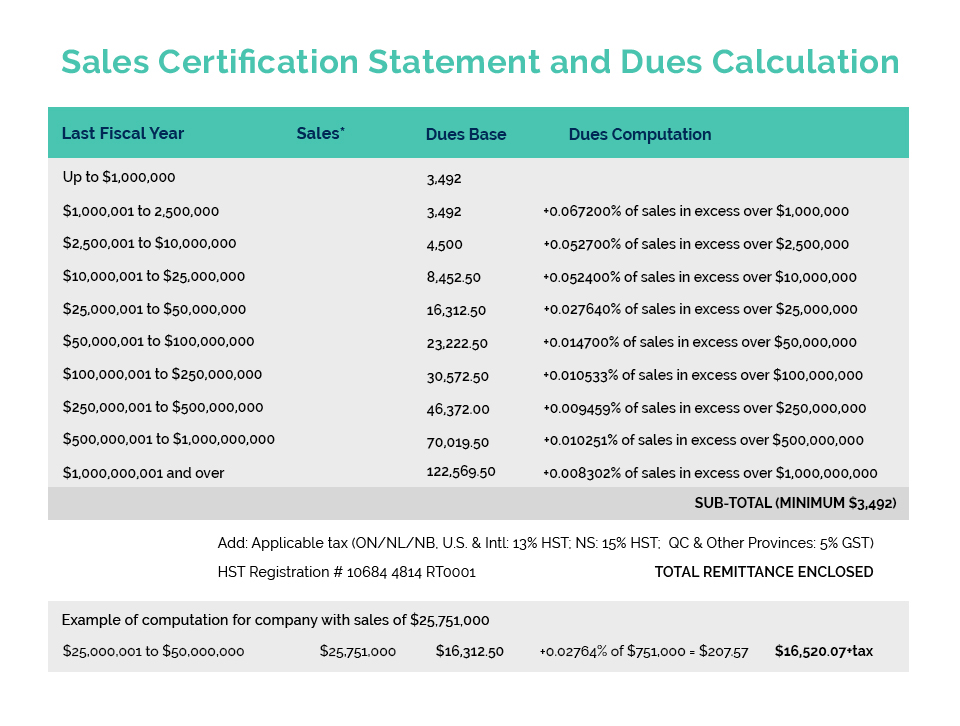
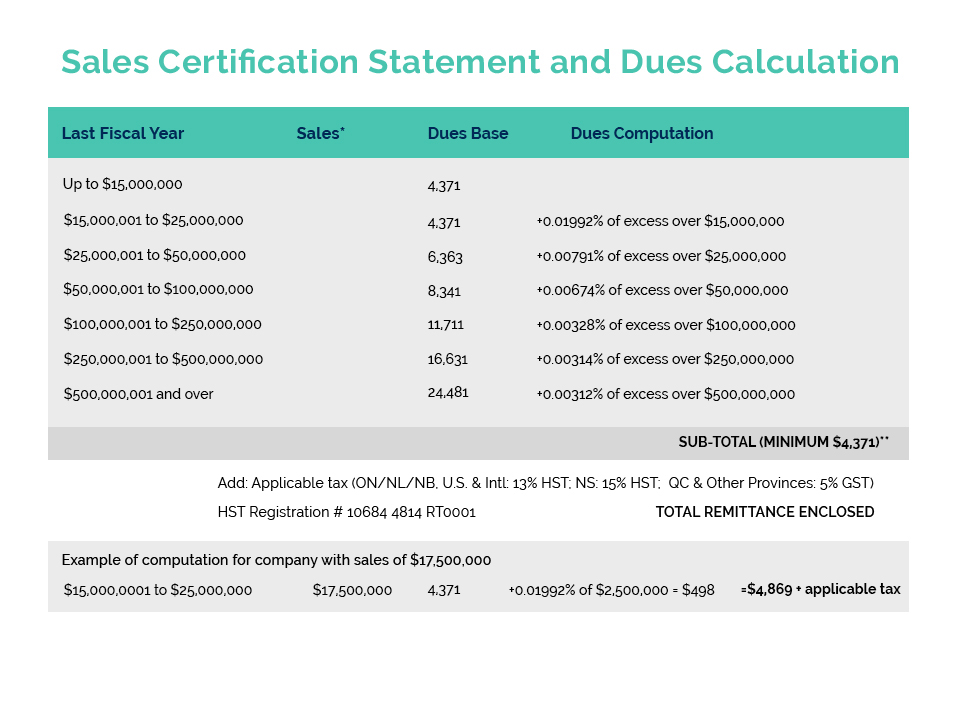
Since the Chinese regulations were amended to allow for an exemption to the requirement for animal testing on imported cosmetics, CA has been pursuing the creation of the necessary government issued GMP which China requires for the exemption. Following extensive work with Health Canada officials on the details, CA has formally submitted a detailed proposal for a voluntary Health Canada GMP Certificate Program, including suggested wording for the certificate which Health Canada would issue (based on various supporting materials and affidavits). Most importantly, our CA proposal was developed with the full participation of HC officials and is expected to receive their supportive recommendation as it progresses through the Departmental approval process. Included in this certificate are the necessary elements to meet the Chinese animal testing exemption. Due to the limited ability for decision making during a federal election, we expect to receive a formal response from HC following the election and confirmation of the Minister for Health.
For background, the requirement for a compliance certificate was created under China’s new cosmetic regulations to allow for the exemption of imported cosmetic products from China’s animal testing requirements and as such provides a significant opportunity for the Canadian cosmetic manufacturing industry. However, any certificate MUST be issued by the government of the exporting jurisdiction which is not something that is part of the regulatory frameworks for cosmetics anywhere across the globe. GMP compliance certificates issued by third parties – including CA – are not acceptable for this purpose.
Although the Chinese provision was officially implemented on May 1, 2021, neither CA, our member companies, or allied trade associations internationally are aware of any certificate that has yet been accepted by Chinese authorities. In fact, it may actually be the intention of Chinese authorities to make it essentially impossible to meet the exemption in order to bolster domestic Chinese products or force international companies to manufacture in China. Having the Canadian Government, as well as other governments, put this to the test by developing a reasonable and responsible pilot certification program – and to test if it would meet the Chinese requirements and if not, why – would assist in determining if this is a non-tariff barrier to trade for which some trade action could be considered. Canadian government officials are aware of this possibility and have been most helpful with the strategy of putting the Chinese intent to the test.
CA remains hopeful that our proposal will facilitate the creation of a Health Canada GMP Certificate and pilot program that could be tested with Chinese authorities. CA eagerly awaits Health Canada’s comments and will keep members updated on developments.