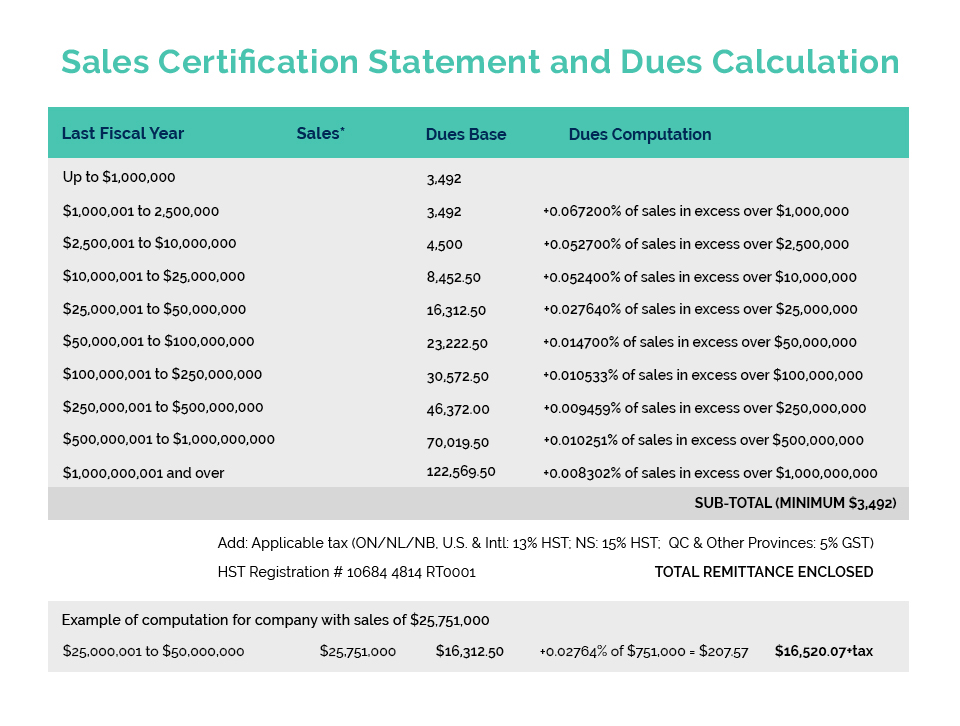
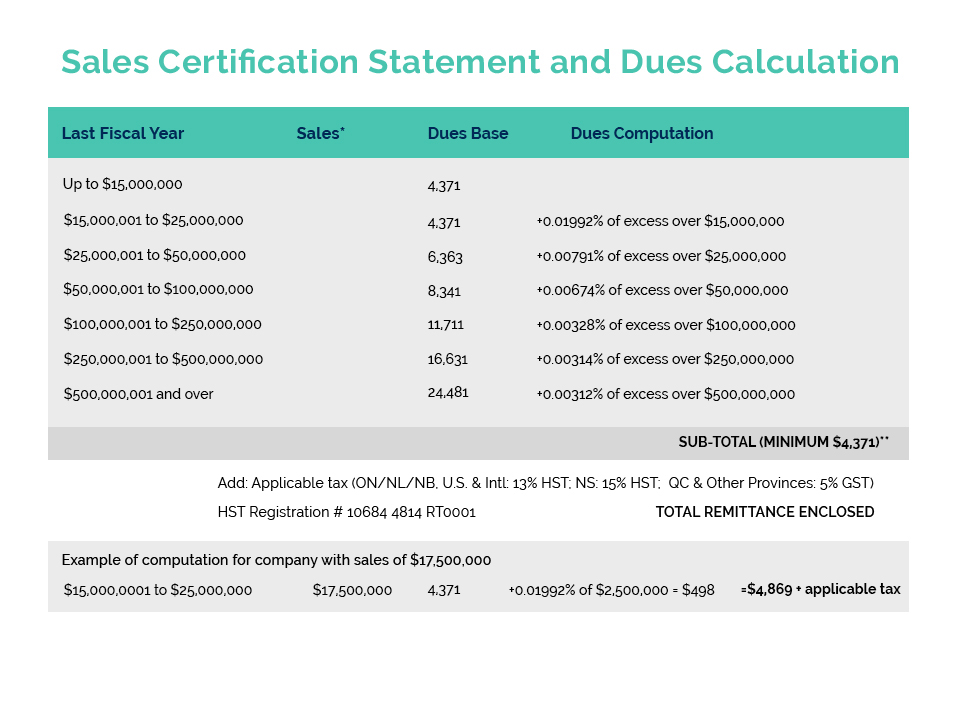
Health Canada recently launched Phase 1 of the implementation of the long awaited Self-Care Framework with the “pre-publication” in Canada Gazette Part 1 of a proposal to amend the Natural Health Product Regulations with respect to labelling. This step is required to ensure the alignment of labelling requirements for the various risk categories under the SCF – whether the products be cosmetics, drugs, or NHP’s – which is a key principle of the SCF. Due to the request of several stakeholder associations, the consultation period that opened on June 26, has now been extended from September 4 to September 24, 2021. CA indicated its support for this limited extension given that the consultation was occurring during the summer holiday period and the call of the federal election.
As members are well aware, CA is very supportive of the launch of Phase 1 as it represents the first step towards finally implementing the Self-Care Framework which is a major and much needed regulatory modernization initiative for the cosmetics and personal care products industry! The Framework will treat “like” products (whether they be cosmetics, drugs, or natural health products) the same, regulating them commensurate with their safety/risk profile. The Government’s materials related to the Phase 1 consultation can be found on CA’s member website in our July communication found HERE.
This NHP Labelling proposal is intended to deliver upon the government’s long- standing commitment to improve labelling for health products while ensuring the alignment of labelling requirements for similar risk products within the Self-Care Framework. Implementation of Phase 2 of the Framework, which will include the further regulatory and legislative changes required for the overall Framework, is expected to be launched in the Spring of 2022.
CA’s Product Compliance & Market Access (PCMA) and Facility Compliance & Manufacturing (FCM) Committees have reviewed the proposal and are providing input to shape CA’s written submission on behalf of our industry. Our comments will include the need to expand the use of digital labels, such as SmartLabel, and allow for suitably implementation periods.