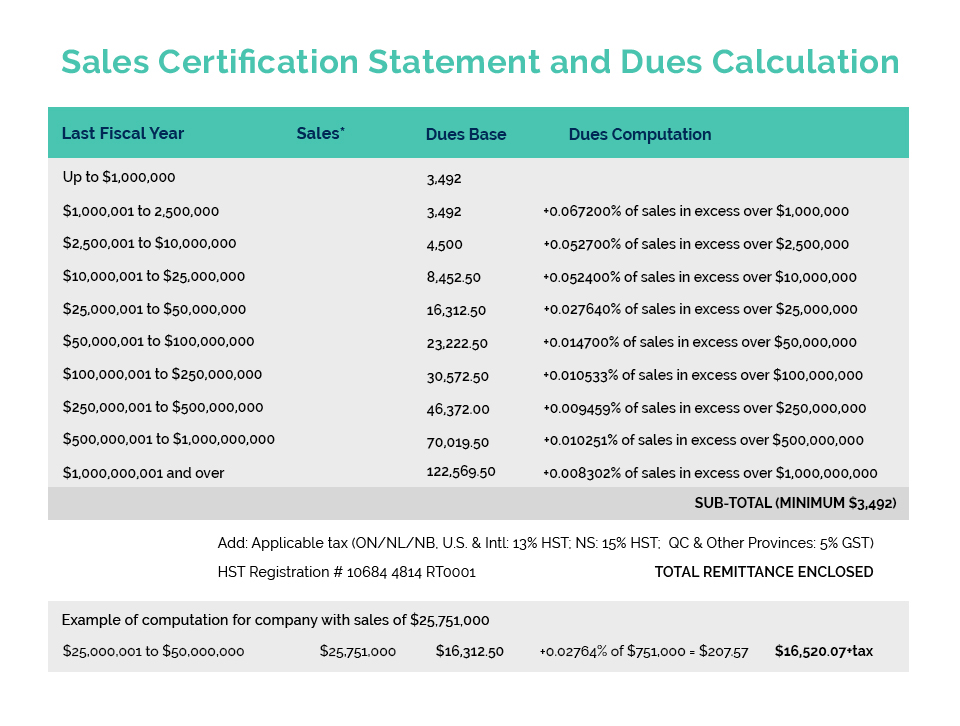
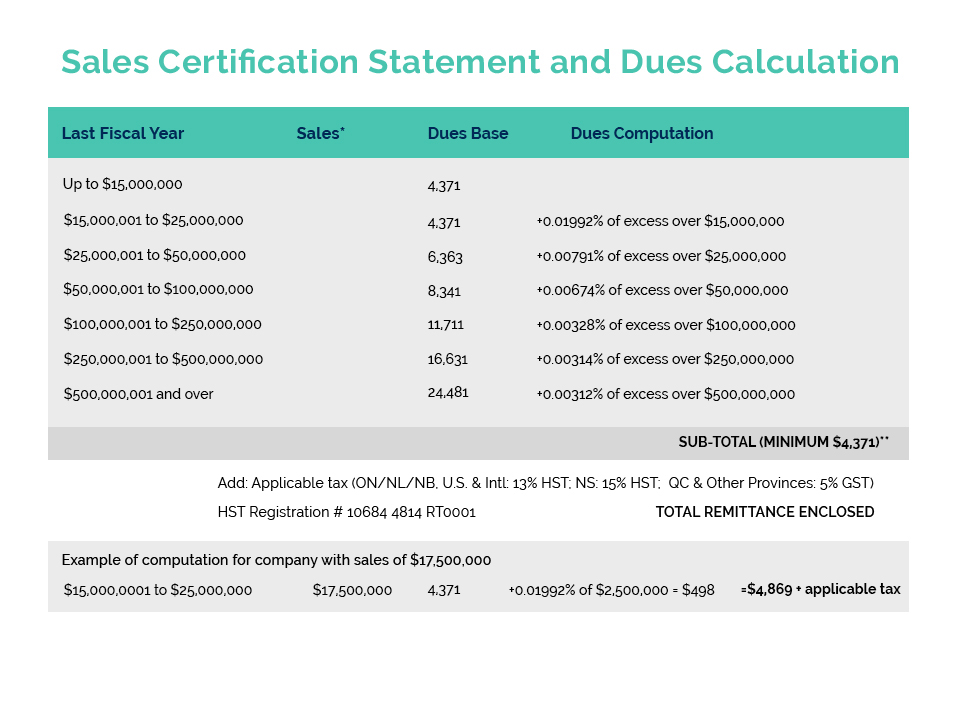
CA’s Product Compliance & Market Access (PCMA) and Facility Compliance & Manufacturing (FCM) Committees met last week to begin the process of preparing for public consultations on Health Canada’s NHP labelling proposals.
According to Health Canada’s forward regulatory plan which was released earlier in the year, the NHP labelling proposals are expected to be pre-published in Part 1 of the Canada Gazette shortly and will launch the formal consultation process for what is to be the first phase of the implementation of the long-awaited Self-Care Framework. Phase 2, which includes the further regulatory changes required for the Framework, will be finalized over the fall/winter and are expected to begin the approval process in the spring of 2022 along with the changes to the Food & Drugs Act that will also be required.
Changes to NHP labelling are being proposed to meet the governments’ commitment to improved labelling for health products, and to ensure the alignment of labelling requirements within the Self-Care Framework. Changing the labelling requirements for non-prescription drugs, which was undertaken a few years ago, proved to be a difficult process as many of the implications – including the potential for larger packaging to accommodate new label requirements – became apparent and led to the development of many new flexibilities including the recognition and use of digital labels. It is expected that the lessons learned from this process will be applied to the new NHP initiative. CA has already sought assurance that such considerations as the expanded use of digital labels (to prevent the need to change package sizes) and long implementation periods (to fit within normal cycles for label changes) will be utilized.
CA expects that this process will focus on NHP labelling within the context of the Self-Care Framework, which will lead to any required adjustments to the labelling of non-prescription drugs to ensure alignment under the various risk categories of the Framework – including eliminating the current requirement for label reviews of low-risk DIN products. The process is also expected to provide a vehicle to consider the longer-term role of digital labels and allow us to propose how this important option can be further utilized including its expected role in e-commerce.
It is very important that all of our member companies with an interest in shaping these developments get involved in the process. We need your contribution to the work that CA is undertaking to help shape the future of this important area. Please watch for more information in our Newsletter and RegEssentials, or contact us at regulatory@cosmeticsalliance.ca to join our technical committee(s).